THANK YOU FOR SUBSCRIBING

Sparta Systems: A Market Leader in Quality Management Software

Pharmaceutical, biotechnology, and medical companies are under more pressure than ever before. Global competition increased regulatory scrutiny, merger and acquisition activity, and complex supply chains are forcing companies to look for ways to deliver drugs and devices in a more streamlined and efficient way. Additionally, patients have come to be at the center of the healthcare value chain with the increase of biologic and combination products, personalized medicine, and gene therapies. To meet these challenges, companies are partnering with Sparta Systems, a leading provider of quality management system software, that helps companies to streamline and automate quality processes, consolidating systems, and ensure regulatory compliance while improving safety and reducing risk.
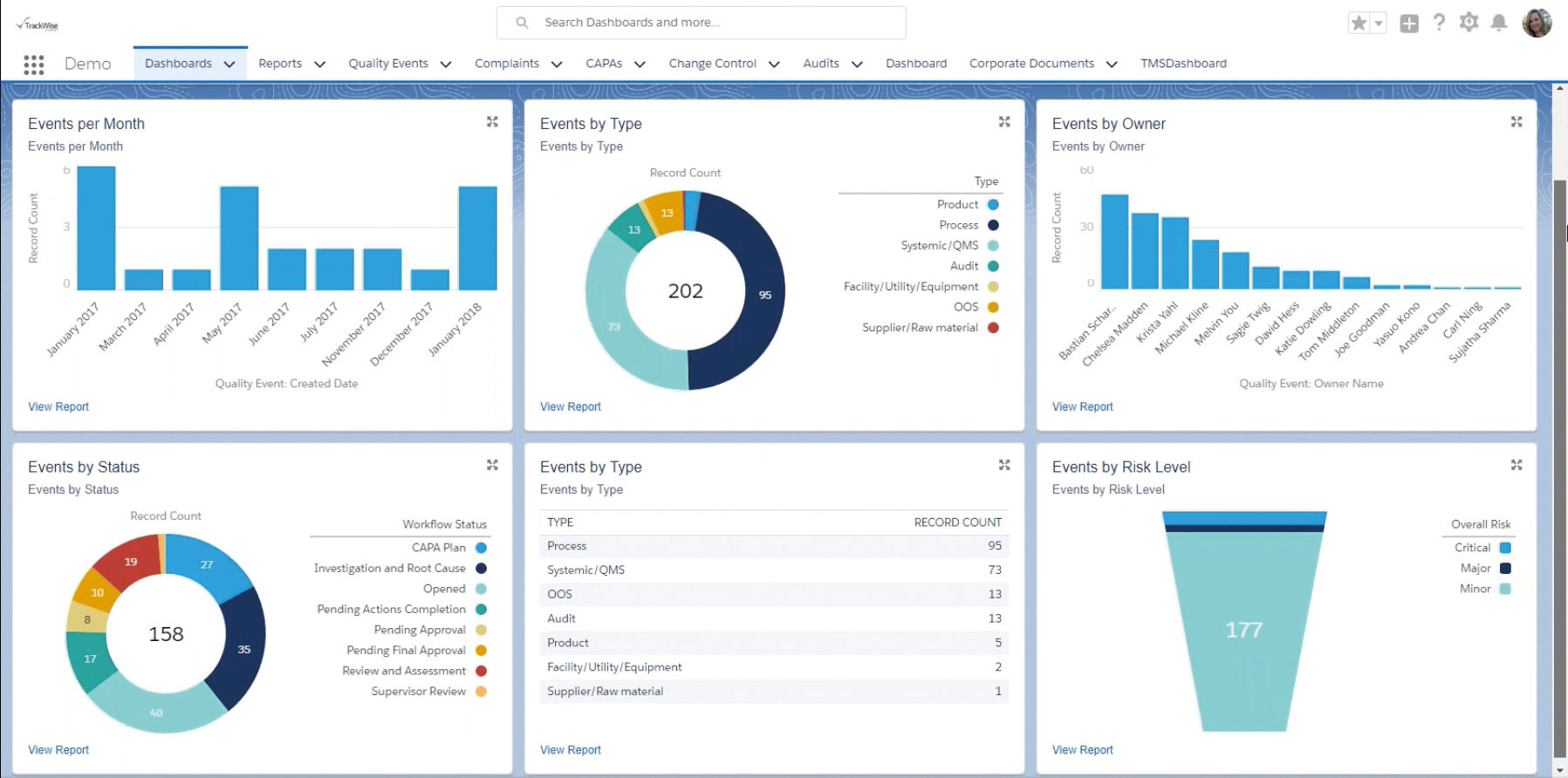
The New Jersey-based firm, founded in 1994, offers two solutions for quality management—TrackWise and TrackWise Digital. Both platforms help companies to automate and streamline their processes, increase efficiency, and meet regulatory demands. TrackWise is an on-premises, integrated quality management solution that automates and streamlines quality processes like audits, CAPAs, change control, complaint handling, document management, training management, and supplier quality management among others. It features robust data and analytics capabilities that can help companies to spot trends and outliers, and support continuous improvement initiatives. Its implementation also helps to decrease companies’ total cost of quality, which can greatly improve companies’ profitability.
Similarly, TrackWise Digital is an easy-to-use integrated quality management system (QMS), only delivered as a subscription service, rather than hosted by the company onsite. By subscribing to this service, companies can lower their total cost of ownership (TCO) for a quality management software system, while reaping the benefits of a traditional enterprise-class solution. Additionally, the solution is pre-validated by Sparta Systems with each new version, reducing the time and resources that regulated companies have historically needed to commit to system revalidation. Moreover, TrackWise Digital’s unique architecture allows companies to choose when new updates are applied to their instance, allowing companies to implement these enhancements at the time that is most convenient for them.
TrackWise Digital has been enormously successful as the company has managed to quadruple the number of clients on the platform over the course of the past two years, with deployments that range from a handful of users to several thousands of users. Additionally, as life sciences companies have sought to integrate industry 4.0 technologies like cloud computing, artificial intelligence, and smart sensors, TrackWise Digital has proven a natural fit as it can easily integrate with other systems and technologies throughout the value chain. In short, TrackWise Digital can help quality management teams to address traditional challenges like product quality, safety, efficacy, compliance and supply chain continuity, while supporting the transformation within manufacturing that is already underway.
The New Jersey-based firm, founded in 1994, offers two solutions for quality management—TrackWise and TrackWise Digital. Both platforms help companies to automate and streamline their processes, increase efficiency, and meet regulatory demands. TrackWise is an on-premises, integrated quality management solution that automates and streamlines quality processes like audits, CAPAs, change control, complaint handling, document management, training management, and supplier quality management among others. It features robust data and analytics capabilities that can help companies to spot trends and outliers, and support continuous improvement initiatives. Its implementation also helps to decrease companies’ total cost of quality, which can greatly improve companies’ profitability.
Similarly, TrackWise Digital is an easy-to-use integrated quality management system (QMS), only delivered as a subscription service, rather than hosted by the company onsite. By subscribing to this service, companies can lower their total cost of ownership (TCO) for a quality management software system, while reaping the benefits of a traditional enterprise-class solution. Additionally, the solution is pre-validated by Sparta Systems with each new version, reducing the time and resources that regulated companies have historically needed to commit to system revalidation. Moreover, TrackWise Digital’s unique architecture allows companies to choose when new updates are applied to their instance, allowing companies to implement these enhancements at the time that is most convenient for them.
TrackWise Digital has been enormously successful as the company has managed to quadruple the number of clients on the platform over the course of the past two years, with deployments that range from a handful of users to several thousands of users. Additionally, as life sciences companies have sought to integrate industry 4.0 technologies like cloud computing, artificial intelligence, and smart sensors, TrackWise Digital has proven a natural fit as it can easily integrate with other systems and technologies throughout the value chain. In short, TrackWise Digital can help quality management teams to address traditional challenges like product quality, safety, efficacy, compliance and supply chain continuity, while supporting the transformation within manufacturing that is already underway.
Sparta Systems is among the first pure-play quality management system software vendors in the market
Customer Success Story
Late last year, the company announced that it had partnered with C-RAD, a rising medical device provider based out of Stockholm, Sweden. C-RAD first approached Sparta when it recognized that the manual effort required to manage their quality records and processes was creating a bottleneck that inhibited the flow of information from one process to the next. Managing quality processes via email, paper and spreadsheets were cumbersome, inefficient, and error-prone.
According to Daniel Pederson, Quality & Regulatory Manager of C-RAD, the implementation of TrackWise Digital was, “Extremely structured, easy to follow and exceeded my expectation. It only took ten weeks to implement process configuration, testing, and validation for complaint handling and CAPA Management. Now the QMS system helps us respond more quickly to issues, which further enhances the safety and security of our products.”
Additionally, C-RAD has plans to use the solution as a foundation for creating IoT-enabled offerings for their customers in the future. By leveraging TrackWise Digital, C-RAD believes it will be able to integrate autonomous product feedback into their quality management system, allowing the company to predict when there is a high probability of product or component failure. Such foresight could then launch preventive actions from the company that can increase customer satisfaction and value.
In short, Sparta is a company that is driven to help its clients—many of whom are the world’s leading pharmaceutical, biotechnology, and medical device companies—to transform the lives of their patients by allowing them to focus on delivering the next wave of high-quality products, services, and experiences. This vision, along with the company’s passion for innovation and willingness to challenge the status quo are what make Sparta the company to partner with along your digital quality journey.

I agree We use cookies on this website to enhance your user experience. By clicking any link on this page you are giving your consent for us to set cookies. More info